Publications

Davies, D. J.; Lahdenperӓ, A. S. K.;.; Phipps, R. J. Catalytic Deracemization of 1,2-Aminoalcohols through Enantioselective Hydrogen Atom Abstraction J. Am. Chem. Soc. 2025, ASAP

Sharif, H.; McCall, L. A.; Sanders, M. A.; Phipps, R. J. Atroposelective Suzuki–Miyaura Coupling to Form 2-Amino-2′-Hydroxybiphenyls Enabled by sRuPhos Angew. Chem. Int. Ed. 2025, Early View

Dolezel, J.; Phipps, R. J. The development of sulfonated terpyridine ligands for control of regioselectivity in palladium-catalysed fluorination of anilides Chem. Sci. 2025, 16, 19694-19701

Adams, H. K.; Fanourakis, A. F.; Dahiya, A.; Băltărețu, I.; Phipps, R. J. Allylic Amination of Alkenyl Alcohols: Simultaneous Control of Chemoselectivity and Enantioselectivity in Nitrene Transfer Using Ion-Paired Catalysts ACS Catal. 2025, 15, 14639–14646.
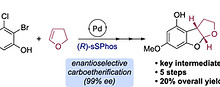
Kadarauch, M.; Phipps, R. J. Enantioselective Formal Synthesis of (−)-Aflatoxin B2 Enabled by Pd-Catalyzed Carboetherification of 2,3-Dihydrofuran Org. Lett. 2025, 27, 8344-8348.

Lit, A. R.^; Takano, S. T.^; Zachau, C.; Băltărețu, I.; Phipps, R. J. Asymmetric Aziridination of Allylic Carbamates Using Ion-Paired Rhodium Complexes and Extrapolation to C─H Amination of Phenethyl Carbamates Angew. Chem. Int. Ed. 2025, 125, e202507532. (^ Equal contribution)

Adams, H. K.^; Kadarauch, M.^; Hodson, N. J.; Lit, A. R.; Phipps, R. J. Design Approaches That Utilize Ionic Interactions to Control Selectivity in Transition Metal Catalysis Chem. Rev. 2025, 125, 2846–2907. (^ Equal contribution)

Kadarauch, M.; Moss, T. A.; Phipps, R. J. Intermolecular Asymmetric Arylative Dearomatization of 1-Naphthols J. Am. Chem. Soc. 2024, 146, 34970-34978.
Lam, N. Y. S.; Dhankhar, J., Lahdenperӓ, A. S. K.; Phipps, R. J., Catalytic Enantioselective Hydrogen Atom Abstraction Enables the Asymmetric Oxidation of Meso Diols J. Am. Chem. Soc. 2024, 146, 33302-33308.


Lahdenperӓ, A. S. K.; Dhankhar, J.^; Davies, D. J.^; Lam, N. Y. S.; Bacoș, P. D.; de la Vega-Hernández, K.; Phipps, R. J., A Chiral Hydrogen Atom Abstraction Catalyst for the Enantioselective Epimerization of Meso Diols. Science 2024, 386, 42-49. (^ Equal contribution)

Hodson, N. J.; Takano, S.; Fanourakis. A.; Phipps, R. J. Enantioselective Nitrene Transfer to Hydrocinnamyl Alcohols and Allylic Alcohols Enabled by Systematic Exploration of the Structure of Ion-Paired Rhodium Catalysts J. Am. Chem. Soc. 2024, 146, 22629–22641

Paterson, K. J.^ ; Dahiya, A.^ ; Williams, B. D.; Phipps, R. J. Tertiary Amides as Directing Groups for Enantioselective C-H Amination using Ion-Paired Rhodium Complexes Angew. Chem. Int. Ed. 2024, e202317489 (^ Equal contribution)

Docherty, P. J.; Kadarauch, M.; Mistry, N.; Phipps, R. J. Application of sSPhos as a Chiral Ligand for Palladium-Catalyzed Asymmetric Allylic Alkylation Org. Lett. 2024, 26, 2862–2866 (Contribution to Organic Chemistry Driven by Academic-Industrial Collaborations special issue)






Kadarauch, M.; Whalley, D. M.; Phipps, R. J. sSPhos: A General Ligand for Enantioselective Arylative Phenol Dearomatization via Electrostatically-Directed Palladium Catalysis J. Am. Chem. Soc. 2023, 145, 25553–25558.
Fanourakis, A.; Phipps, R. J. Catalytic, asymmetric carbon–nitrogen bond formation using metal nitrenoids: from metal–ligand complexes via metalloporphyrins to enzymes Chem. Sci. 2023, 14, 12447-12476
Ermanis, K.*; Gibson, D. C.; Genov, G. R.; Phipps, R. J.* Interrogating the Crucial Interactions at Play in the Chiral Cation-Directed Enantioselective Borylation of Arenes ACS Catal. 2023, 13, 13043-13055
Gillespie, J. E.; Lam, N. Y. S.; Phipps, R. J. Ortho-Selective Amination of Arene Carboxylic Acids via Rearrangement of Acyl O-Hydroxylamines Chem. Sci. 2023 14, 10103-10111
Bacoș, P. D.^; Lahdenperӓ, A. S. K.^; Phipps, R. J Discovery and Development of the Enantioselective Minisci Reaction Acc. Chem. Res. 2023, 56, 2037-2049 (^ Equal contribution)
Fanourakis, A.; Hodson, N.; Lit, A. R. and Phipps, R. J. Substrate-Directed Enantioselective Aziridination of Alkenyl Alcohols Controlled by a Chiral Cation J. Am. Chem. Soc. 2023, 145, 7516–7527













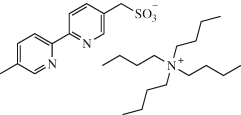
















Lahdenperӓ, A. S. K.; Bacoș, P. D.; Phipps, R. J. Enantioselective Giese Additions of Prochiral α-Amino Radicals J. Am. Chem. Soc. 2022, 144, 22451-22457.
Gillespie, J. E.;^ Fanourakis, A. F.;^ Phipps, R. J. Strategies That Utilize Ion Pairing Interactions to Exert Selectivity Control in the Functionalization of C–H Bonds J. Am. Chem. Soc., 2022, 144, 18195-18211 (^ Equal contribution)
Pearce-Higgins, R.;^ Hogenhout, L. N.; ^ Docherty, P. J.; ^ Whalley, D.M.; Chuentragool, P.; Lee, N.; Lam, N. Y. S.; McGuire, T.M.; Valette, D.; Phipps, R. J. An Enantioselective Suzuki-Miyaura Coupling to Form Axially Chiral Biphenols J. Am. Chem. Soc., 2022, 144, 15026-15032
(^ Equal contribution)
Morrill, C.; Gillespie, J. E.; Phipps, R. J. An Aminative Rearrangement of O-(Arenesulfonyl)hydroxylamines: Facile Access to ortho-Sulfonyl Anilines Angew. Chem. Int. Ed. 2022, 61, e202204025
Douthwaite, J. L.; Phipps, R. J. Extended Sulfonated Bipyridine Ligands Targeting the Para-Selective Borylation of Arenes Tetrahedron, 2022, 117, 132831
(Invited contribution for special issue in honour of Professor Franziska Schoenebeck on her receipt of the 2022 Tetrahedron Young Investigator Award.)
Colgan, A. C.; Proctor, R. S. J.; Gibson, D. C.; Chuentragool, P.; Lahdenperӓ, A. S. K.; Ermanis, K.;* Phipps, R. J.* Hydrogen Atom Transfer Driven Enantioselective Minisci Reaction of Alcohols Angew. Chem. Int. Ed., 2022, 61, e202200266
Fanourakis, A.; Williams, B. D.; Paterson, K. J.; Phipps, R. J. Enantioselective Intermolecular C-H Amination Directed by a Chiral Cation J. Am. Chem. Soc., 2021 143, 10070–10076
Gillespie, J. E.; Morrill, C.; Phipps, R. J. Regioselective Radical Arene Amination for the Concise Synthesis of ortho-Phenylenediamines J. Am. Chem. Soc., 2021, 143, 9355–9360
Proctor, R. S. J.; Chuentragool, P.; Colgan, A. C.; Phipps, R. J. Hydrogen Atom Transfer-Driven Enantioselective Minisci Reaction of Amides J. Am. Chem. Soc., 2021, 143, 4928-4934
Golding, W. A.^; Schmitt, H. L.^; Phipps, R. J. Systematic Variation of Ligand and Cation Parameters Enables Site-Selective C-C and C-N Cross Coupling of Multiply-Chlorinated Arenes Through Substrate-Ligand Electrostatic Interactions. J. Am. Chem. Soc., 2020, 142, 21891–21898 (^Equal contribution)
Ermanis, K.;* Colgan, A. C.; Proctor, R. S. J. ; Hadrys, B. W.; Phipps, R. J.;* Goodman, J. M.* A Computational and Experimental Investigation of the Origin of Selectivity in the Chiral Phosphoric Acid Catalyzed Enantioselective Minisci Reaction. J. Am. Chem. Soc. 2020,142, 21091–21101.
Feature article in Trends in Chemistry (Mechanism of the Month feature):
Colgan, A. C.; Phipps, R. J. Catalytic Enantioselective Minisci Reaction, Trends Chem. 2020 In Press
Proctor, R. S. J.; Colgan, A. C.; Phipps, R. J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates, Nature Chemistry, 2020, 12, 990-1004.
Fanourakis, A.; Docherty, P.J.; Chuentragool, P.; Phipps, R.J. Recent Developments in Enantioselective Transition Metal Catalysis Featuring Attractive Noncovalent Interactions between Ligand and Substrate, ACS Catalysis, 2020, 10, 10672-10714
Genov, G. R.; Douthwaite, J. L.; Phipps, R. J. 5′‐Methyl[2,2′‐bipyridine]‐5‐methanesulfonic Acid, Tetrabutylammonium Salt eEROS, Encyclopedia of Reagents for Organic Synthesis, Wiley, 2020
Hadryš, B.; Phipps, R. J. Acid and Solvent Effects on the Regioselectivity of Minisci-Type Addition to Quinolines Using Amino Acid Derived Redox Active Esters Synlett, 2020, just accepted. Invited contribution to Cluster on Heterocyclic Chemistry.
Genov, G. R.^; Douthwaite, J. L.^; Lahdenperä, A.S.K.; Gibson, D. C.; Phipps, R. J. Enantioselective remote C–H activation directed by a chiral cation Science, 2020, 367, 1246-1251
(^Equal contribution)
Highlighted in: Synfact of the Month "Chiral Cation–Anion Ligand Pair Directs Highly Selective Remote C–H Activation"
Golding, W. A.; Phipps, R. J. Electrostatically-Directed Pd-Catalysis in Combination with C-H Activation: Site-Selective Coupling of Remote Chlorides with Fluoroarenes and Fluoroheteroarenes
Chem. Sci. 2020, 11, 3022-3027.
Reid, J. P.^; Proctor, R. S. J.^; Sigman, M. S.*; Phipps, R. J.* Predictive Multivariate Linear Regression Analysis Guides Successful Catalytic Enantioselective Minisci Reactions of Diazines
J. Am. Chem. Soc. 2019, 141, 19178-19185
(^Equal Contribution)
Mihai, M. T.; Williams, B. D.; Phipps, R. J. Para-Selective C-H Borylation of Common Arene Building Blocks Enabled by Ion-Pairing with a Bulky Countercation J. Am. Chem. Soc. 2019, 141, 15477-15482
Highlighted in: Chemistry World "Steric shield leads boron to aromatic rings’ most remote region"
Lee, B.; Mihai, M. T.; Stojalnikova, V.; Phipps, R. J. Ion Pair-Directed Borylation of Aromatic Phosphonium Salts (Invited Contribution to Special Issue on C-H Functionalization) J. Org. Chem. 2019, 84, 13124-13134
Proctor, R. S. J.; Phipps, R. J. Recent Advances in Minisci-Type Reactions Angew. Chem. Int. Ed. 2019 58, 13666-13699
Golding, W. A.; Pearce-Higgins, R.; Phipps, R. J. Site-Selective Cross-Coupling of Remote Chlorides Enabled by Electrostatically-Directed Palladium Catalysis J. Am. Chem. Soc. 2018, 140, 13570–13574.
Proctor, R. S. J.; Davis, H. J.; Phipps, R. J. Catalytic enantioselective Minisci type addition to heterorenes Science 2018, 360, 419-422 .
Highlighted in: C&E News "Radical new way to control chirality"
Chemistry World "Making light work of synthesis"
Mihai, M. T.; Davis, H. J.; Genov, G. R.; Phipps, R. J. Ion Pair-Directed C-H Activation on Flexible Ammonium Salts: Meta-Selective Borylation of Quaternized Phenethylamines and Phenylpropylamines ACS Catalysis 2018, 8, 3764-3769.
Mihai, M. T.; Genov, G. R.; Phipps, R. J. Access to the meta position of arenes through transition metal catalysed C–H bond functionalisation: a focus on metals other than palladium (2018 Emerging Investigators Special Issue) Chem. Soc. Rev. 2018, 47, 149-171.
Türtscher, P. L.; Davis, H. J.; Phipps, R. J.; Palladium-Catalysed Cross-Coupling of Benzylammonium Salts with Boronic Acids under Mild Conditions (Invited Contribution for Bürgenstock Special Section 2017: Future Stars in Organic Chemistry) Synthesis 2018, 50, 793-802.
Davis, H. J.; Genov, G. R.; Phipps, R. J. Meta Selective C-H Borylation of Benzylamine, Phenethylamine and Phenylpropylamine-Derived Amides Enabled by a Single Anionic Ligand Angew. Chem. Int. Ed. 2017, 56, 13351-13355.
Montenegro, J.; Phipps, R. J.; Highlights from the 52nd EUCHEM conference on stereochemistry, Bürgenstock, Switzerland, May 2017 Chem. Commun. 2017, 53, 9960-9966.
Mihai, M. T.; Phipps, R. J.; Ion-Pair-Directed meta-Selective C–H Borylation of Aromatic Quaternary Ammonium Salts (Synpacts Account) Synlett 2017, 28, 1011-1017.
Davis, H. J.; Phipps, R. J.; Harnessing Non-Covalent Interactions to Exert Control Over Regioselectivity and Site-Selectivity in Catalytic Reactions Chem. Sci. 2017, 8, 864-877.
Davis, H. J.; Mihai, M. T.; Phipps, R. J.; Ion Pair-Directed Regiocontrol in Transition Metal Catalysis: A Meta-Selective C–H Borylation of Aromatic Quaternary Ammonium Salts J. Am. Chem. Soc. 2016, 138, 12759-12762.
Post-Doc
15.
Beaud, R.; Phipps, R. J.; Gaunt, M.J. Enantioselective Cu-Catalyzed Arylation of Secondary Phosphine Oxides with Diaryliodonium Salts toward the Synthesis of P-Chiral Phosphines. J. Am. Chem. Soc. 2016, 138, 13183-13186.
14.
Yang, X.; Wu, T.; Phipps, R. J.; Toste, F. D.; Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826-870.
13.
12.
Yang, X.*; Phipps, R. J.*; Toste, F. D. Asymmetric Fluorination of a-Branched Cyclohexanones Enabled by a Combination of Chiral Anion Phase-Transfer Catalysis and Enamine Catalysis using Protected Amino Acids. J. Am. Chem. Soc. 2014, 136, 5225-5528 *(Authors contributed equally).
11.
Wu, J.; Wang, Y.; Drljevic, A.; Rauniyar,V.; Phipps, R. J.; Toste, F. D. A combination of directing groups and chiral anion phase-transfer catalysis for enantioselective fluorination of alkenes. Proc. Natl. Acad. Sci. USA 2013, 110, 13729-13733
10.
Phipps, R. J.; Toste, F. D. Chiral anion phase-transfer catalysis applied to the direct enantioselective fluorinative dearomatization of phenols J. Am. Chem. Soc. 2013, 135, 1268-1271.
9.
Honjo, T.; Phipps, R. J.; Rauniyar, V.; Toste, F. D. A doubly axially chiral phosphoric acid catalyst for the asymmetric tandem oxyfluorination of enamides. Angew. Chem. Int. Ed. 2012, 51, 9684-9688.
8.
Phipps, R. J.; Hamilton, G. L.; Toste, F. D. The progression of chiral anions from concepts to applications in asymmetric catalysis. Nature Chem. 2012, 4, 603-614.
7.
Phipps, R. J.; Hiramatsu, K.; Toste, F. D. Asymmetric fluorination of enamides: access to a-fluoroimines using an anionic chiral phase-transfer catalyst J. Am. Chem. Soc. 2012, 134, 8376-8379.
PhD
6.
Phipps, R. J.; McMurray, L. M.; Duong, H. A.; Ritter, S.; Gaunt, M. J. Copper-catalyzed alkene arylation with diaryliodonium salts. J. Am. Chem. Soc. 2012, 134, 10773-10776.
5.
Duong, H. A.; Gilligan, R. E.; Cooke, M. L.; Phipps, R. J.; Gaunt, M. J. Copper(II)-catalyzed meta-selective direct arylation of a-aryl carbonyl compounds. Angew. Chem. Int. Ed. 2011, 50, 463-466.
4.
Ciana, C. L.; Phipps, R. J.; Brandt, J. R.; Magnus, F. –M.; Gaunt, M. J. A highly para-selective copper(II)-catalyzed direct arylation of aniline and phenol derivatives. Angew. Chem. Int. Ed. 2011, 50, 458-462.
3.
Phipps, R. J.; Gaunt, M. J. A meta-selective copper-catalyzed C-H bond arylation. Science 2009, 323, 1593-1597.
2.
Phipps, R. J.; Grimster, N. P.; Gaunt, M. J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 2008, 130, 8172-8174.
Undergraduate
1.
Paddock, V.L.; Phipps, R. J.; Conde-Angulo, A.; Blanco-Martin, A.; Giró-Mañas, C.; Martin, L. J.; White, A. J. P.; Spivey, A. C. (±)-trans,cis-4-Hydroxy-5,6-di-O-isopropylidenecyclohex-2-ene-1-one: synthesis and facile dimerization to decahydrodibenzofurans. J. Org. Chem. 2011, 76, 1483-1486.
